Amino Acids Are Joined By
19.one: Polypeptides and Proteins
- Page ID
- 3426
Learning Objectives
- Ascertain or describe the following:
- amino acid
- "R" grouping
- peptide bond
- peptide
- polypeptide
- primary protein structure
- secondary poly peptide structure
- tertiary protein structure
- fourth protein structure
- gene
- Depict how the main structure of a protein or polypeptide ultimately detemines its final three-dimensional shape.
- Draw how the order of nucleotide bases in Dna ultimately determines the final 3-dimensional shape of a protein or polypeptide.
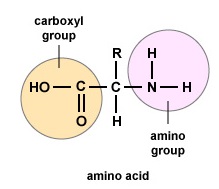
Amino acids are the building blocks for proteins. All amino acids comprise an amino or NH2 grouping and a carboxyl (acid) or COOH group. There are twenty unlike amino acids commonly constitute in proteins and often 300 or more amino acids per poly peptide molecule. Each amino acid differs in terms of its "R" group. The "R" grouping of an amino acid is the remainder of the molecule, that is, the portion other than the amino grouping, the acrid group, and the central carbon. Each unlike amino acid has a unique "R" group and the unique chemical properties of an amino acid depend on that of its "R" group (Effigy \(\PageIndex{1}\)).
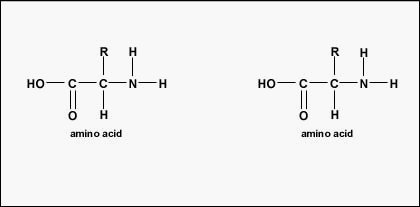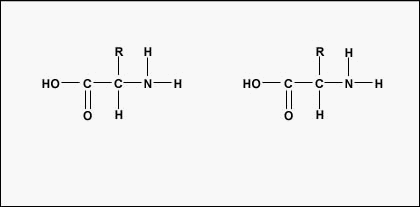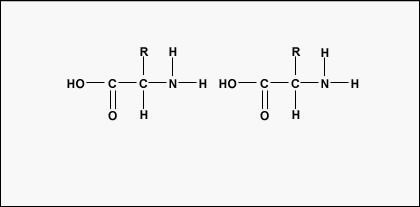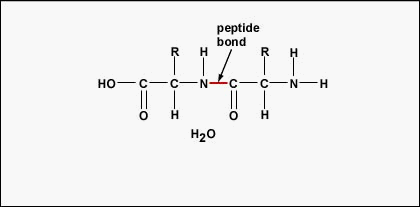
To form polypeptides and proteins, amino acids are joined together by peptide bonds, in which the amino or NH2 of one amino acrid bonds to the carboxyl (acid) or COOH group of another amino acid as shown in (Figure \(\PageIndex{2}\) and Figure \(\PageIndex{3}\)).
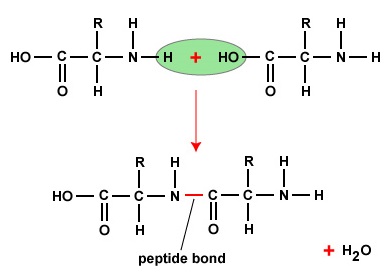
A peptide is two or more amino acids joined together past peptide bonds, and a polypeptide is a concatenation of many amino acids. A protein contains one or more than polypeptides. Therefore, proteins are long chains of amino acids held together by peptide bonds.
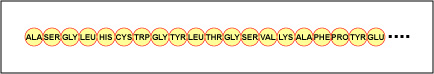
The actual gild of the amino acids in the protein is called its primary construction (Figure \(\PageIndex{four}\)) and is adamant by DNA. As will be seen afterward in this unit, DNA is divided into functional units chosen genes. A gene is a sequence of deoxyribonucleotide bases along one strand of Dna that codes for a functional product - a specific molecule of messenger RNA, transfer RNA, or ribosomal RNA. The product is usually messenger RNA (mRNA) and mRNA ultimately results in the synthesis of a polypeptide or a poly peptide. Therefore, it is commonly said that the order of deoxyribonucleotide bases in a gene determines the amino acid sequence of a detail poly peptide. Since certain amino acids can interact with other amino acids in the same poly peptide, this main structure ultimately determines the final shape and therefore the chemical and concrete properties of the protein.
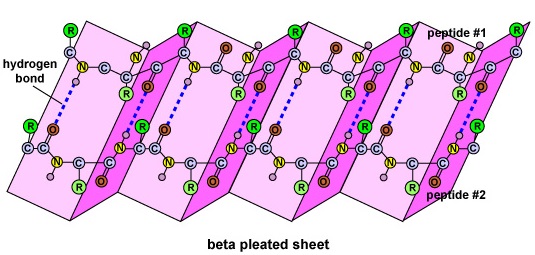
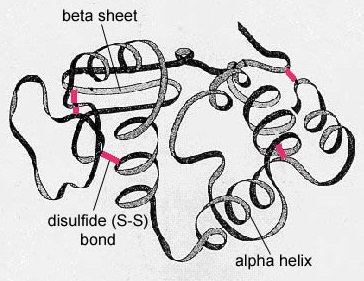
The secondary construction of the poly peptide is due to hydrogen bonds that grade between the oxygen atom of ane amino acid and the nitrogen atom of another. This gives the poly peptide or polypeptide the two-dimensional form of an alpha-helix or a beta-pleated sheet (Effigy \(\PageIndex{5}\)).
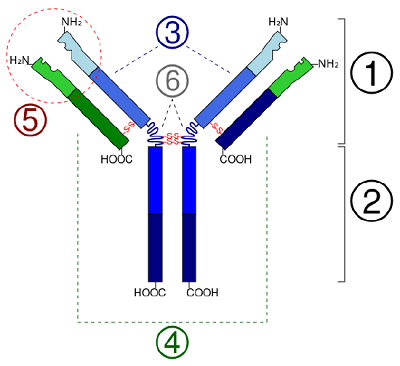
In globular proteins such as enzymes, the long chain of amino acids becomes folded into a 3-dimensional functional shape or tertiary structure. This is considering certain amino acids with sulfhydryl or SH groups class disulfide (S-Due south) bonds with other amino acids in the aforementioned chain. Other interactions between R groups of amino acids such every bit hydrogen bonds, ionic bonds, covalent bonds, and hydrophobic interactions too contribute to the third structure (Figure \(\PageIndex{6}\)). In some proteins, such as antibody molecules and hemoglobin, several polypeptides may bond together to grade a quaternary structure (Effigy \(\PageIndex{vii}\)).
As volition be seen afterwards in this unit, during protein synthesis, the gild of nucleotide bases forth a gene gets transcribed into a complementary strand of mRNA which is then translated by tRNA into the right guild of amino acids for that polypeptide or poly peptide. Therefore, the club of deoxyribonucleotide bases along the Deoxyribonucleic acid determines the order of amino acids in the proteins. Considering certain amino acids can interact with other amino acids, the social club of amino acids for each protein determines its last three-dimensional shape, which in plough determines the office of that protein (eastward.thou., what substrate an enzyme will react with, what epitopes the Fab of an antibody will combine with, what receptors a cytokine volition bind to).
Summary
- Amino acids are the building blocks for proteins. There are twenty different amino acids ordinarily found in proteins and often 300 or more amino acids per protein molecule.
- All amino acids comprise an amino or NH2 grouping and a carboxyl (acid) or COOH group.
- To class polypeptides and proteins, amino acids are joined together by peptide bonds, in which the amino or NH2 of 1 amino acid bonds to the carboxyl (acid) or COOH group of another amino acid.
- A peptide is ii or more than amino acids joined together past peptide bonds; a polypeptide is a chain of many amino acids; and a protein contains 1 or more than polypeptides. Therefore, proteins are long bondage of amino acids held together past peptide bonds.
- The bodily club of the amino acids in the protein is chosen its primary structure and is determined by Dna.
- The society of deoxyribonucleotide bases in a gene determines the amino acrid sequence of a item protein. Since certain amino acids can interact with other amino acids in the same poly peptide, this primary structure ultimately determines the last shape and therefore the chemic and physical properties of the protein.
- The secondary construction of the protein is due to hydrogen bonds that form between the oxygen atom of one amino acrid and the nitrogen atom of another and gives the poly peptide or polypeptide the two-dimensional form of an alpha-helix or a beta-pleated sheet.
- In globular proteins such as enzymes, the long chain of amino acids becomes folded into a three-dimensional functional shape or tertiary structure. This is considering certain amino acids with sulfhydryl or SH groups form disulfide (Southward-S) bonds with other amino acids in the same concatenation. Other interactions between R groups of amino acids such as hydrogen bonds, ionic bonds, covalent bonds, and hydrophobic interactions besides contribute to the tertiary structure.
- In some proteins, such as antibody molecules, several polypeptides may bond together to form a quaternary structure.
Amino Acids Are Joined By,
Source: https://bio.libretexts.org/Bookshelves/Microbiology/Book:_Microbiology_%28Kaiser%29/Unit_7:_Microbial_Genetics_and_Microbial_Metabolism/19:_Review_of_Molecular_Genetics/19.1:_Polypeptides_and_Proteins
Posted by: urestiboure1963.blogspot.com


0 Response to "Amino Acids Are Joined By"
Post a Comment